Composition and Action Mode of Antibody Drug Conjugate

Composition and Action Mode of Antibody Drug Conjugate
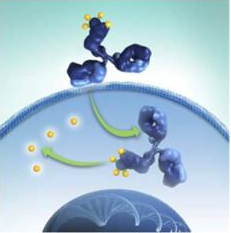
ADC usually consists of three parts: a specific antibody that can target cancer cells, a protein that plays a role in connection and a cytotoxic chemical drug molecule attached to the antibody. After the antibody binds to the specific receptor on the surface of the cancer cell, it will be "swallowed" by the cancer cell and release cytotoxic drugs in the cancer cell to play a killing role.
On the one hand, ADCs are expected to be used in a variety of cancer treatments because the antibody binding to cancer cell targets can be customized. On the other hand, the cytotoxic drugs contained in ADCs are usually unable to be administered alone due to "too much firepower". This mode of action can precisely reduce the damage of cytotoxic drugs to healthy cells.
Such drugs are usually administered by intravenous injection.
Possible side effects
Depending on the targeted cancer cell receptor and the cytotoxic drug contained, the side effects of specific drugs vary. The most common adverse reaction may be that before ADC is swallowed into cancer cells, cytotoxic drugs are released into the blood in advance, resulting in low red blood cell, white blood cell, and platelet counts, peripheral neuropathy, liver damage, and changes in vision.
Current applications in cancer treatment
There are currently five ADCs approved for the treatment of blood cancer or breast cancer.
Mylotarg (Gemtuzumab ozogamicin): has been approved by the US FDA and the European Union for acute myeloid leukemia (AML). The drug targets CD33 receptors expressed on the surface of myeloblasts, and as many as 90% of AML patients have CD33.
Adcetris (Brentuximab vedotin): targeting CD30 receptor, has been approved by the FDA for some patients with classic Hodgkin's lymphoma and anaplastic large cell lymphoma.
Besponsa (Besponsa): Targets the CD22 receptor and has been approved by the FDA for B-cell precursor acute lymphocytic leukemia.
Polivy (Polatuzumab vedotin-piiq): targeting CD79b receptor, has been approved by the FDA in combination with chemotherapy for relapsed diffuse large B-cell lymphoma (the most common non-Hodgkin lymphoma. The FDA has pointed out that this is also the first immunochemotherapy for this disease. In a phase 1b/2 clinical trial, patients receiving Polivy and standard therapies (bendamustine and rituximab) were more than double the complete response rate of the standard treatment group (40% vs 18%).
Kadcyla (ado-trastuzumab emtansine): contains the breast cancer monoclonal antibody trastuzumab, targets HER2 protein, and is approved by the FDA for the adjuvant treatment of HER2-positive early breast cancer and the treatment of metastatic breast cancer.
Prospects for drugs development
At the same time, there are nearly 60 ADCs under study in nearly 600 clinical trials. The new-generation ADC has also improved the drug design of ADC's connexin and cytotoxic drug release mechanism.
Blood cancers including leukemia and lymphoma are still the treatment targets of most ADCs, but more and more new-generation ADCs are beginning to explore breast cancer, colorectal cancer, lung cancer, prostate cancer, ovarian cancer, and urethral epithelial cancer and other solid tumors. For example, at the beginning of the year, an ADC called tisotumab vedotin showed promise in the treatment of six different cancer patients in early clinical trials.
As one of the new immunotherapy drugs and one of the main strategies for combating cancer, ADC is being continuously developed worldwide, and it is expected that the efficacy and safety of more drugs will be verified to benefit more cancer patients.