The Increasingly Important Role of Single Domain Antibodies

The Increasingly Important Role of Single Domain Antibodies
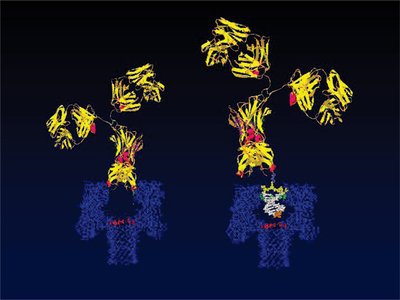
Abstract: In recent years, a variety of monoclonal antibody drugs have been successfully marketed and used to treat a variety of human diseases, but due to the complex structure of monoclonal antibodies and high production costs. Limit its wide clinical application. With advances in biotechnology, antibodies have been developed toward miniaturized genetically engineered single domain antibodies. At the end of the 20th century, a naturally occurring light chain heavy chain antibody (HCAb) was found in camellias and nurses sharks. The antigen-binding site of this particular antibody consisted of only a single domain. This domain is called VHH in camel. It is easy to be expressed by genetic modification. The product is called VHH single domain antibody, which has the advantages of small molecular weight, good solubility, high stability and strong tissue penetration. The application prospects of functions are more extensive. This paper reviews the progress of the research on the application of camel-derived VHH single domain antibody in immunotherapy, and provides a new idea for genetic engineering single domain antibody transformation. Single-domain antibody (sdAbs) is a novel antibody with antigen-binding activity that retains the heavy chain variable region and is cloned from camellia and chondrocyte serum by genetic engineering in recent years. It has the advantages of high specificity, good affinity, high stability, strong tissue penetration, low immunogenicity and low preparation cost. It has achieved certain results in the fields of diagnosis, treatment and detection and has received extensive attention.
Keywords: camel VHH antibody, single domain antibody, heavy chain antibody
Research status of single domain antibody
In 1989, Ward et al. first prepared a genetically engineered antibody consisting only of the VH domain and named it a single domain antibody. They used surface Plasmon resonance to measure the affinity of single-domain antibodies and compared their affinity to their corresponding Fab antibodies. It was found that the affinity of single-domain VH antibodies was about 250-fold lower than that of Fab antibodies. For general antibodies, the heavy and light chain variable regions contribute differently to antigen binding, VH plays a major role, and VH alone still has antigen binding ability, but the lack of VL will reduce the affinity of VH for antigen. And easy to accumulate and precipitate. In recent years, single-domain antibodies constructed by cloning the VH and VL of general antibodies have greatly improved in affinity and stability, but the engineering of such antibodies is not universal. Therefore, single-domain antibodies (VH or VL) with smaller molecules are still suspected in terms of application range and utilization value.
Heavy chain antibody discovery
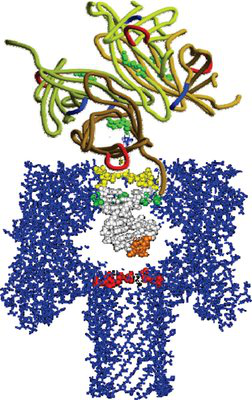
For a long time, we believe that naturally occurring antibodies are composed of two parts, heavy and light. In 1993, Hamers-Casterman et al. reported an antibody that naturally occupies a large amount of light chain in the camellia serum, namely the heavy chain antibody (HCAb, Fig. 1). Heavy chain antibodies play an important role in humeral immunity in camels. In addition to the lack of a light chain, the heavy chain antibody has no CH1 region between its heavy chain variable region and the hinge region, and the VHH of the heavy chain antibody also has a characteristic different from VH. These changes are essential for the function of heavy chain antibodies. Studies have found that in mammals, heavy chain antibodies are currently only found in camels and llamas, except for some heavy chain disease patients in humans that contain antibodies that lack the light chain and are unable to define VH and CH1. The discovery of heavy chain antibodies has provided new ideas for the study of genetically engineered antibodies, and the genetic engineering of heavy chain antibodies has gradually been carried out.
Cloning and expression of camel VHH single domain antibody and its biochemical characteristics
To date, antigen-specific VHH single domain antibodies have been amplified from peripheral blood lymphocytes of immunized or immunized camellias by PCR, and cloned into phage display vectors to screen phage libraries with specific antigens. However, the single-domain antibody derived from the unimmunized pool has a weak affinity with the antigen; so many research groups have obtained a high antigen affinity single domain antibody by constructing an immunological library. The selected VHH single domain antibody is easier to express and purify in E. coli than the traditional antibody, and has high solubility and stability, and can be stably expressed in yeast, plant or mammalian cells. Production costs are greatly reduced. In addition, the VHH antibody obtained by cloning expression has many advantages over the conventional antibody and the single-chain antibody scFv. Such as strong water solubility, high heat resistance and resistance to denaturant treatment. Since there is no need for a synthetic linker like scFv and several hydrophilic amino acids in the conserved sequence of the FR2 region, the VHH single domain antibody has a water-soluble character.
The use of VHH single domain antibody in immunotherapy.
ü Inhibit leukocyte extracellular enzyme activity.
ü Neutralize cytokines and soluble proteins.
ü Targeting intracellular antigens.
ü Tumor targeted immunodiagnostics’ and treatment.
Prospects and future research directions
Since the accidental discovery of heavy chain antibodies in camels, the application of phage display technology to obtain antigen-specific single domain antibodies has become a hot topic. A large number of basic studies have shown that single-domain antibodies derived from heavy chain antibodies can be effectively used in the development of diagnostic reagents and biosensors, and have great diagnostic and therapeutic prospects. The most interesting issue is the single-domain antibody as a clinical therapeutic drug, and long-term repeated clinical use can produce potential immunogenicity. The immunogenicity of proteins increases with molecular size and complexity. Systemic small doses of single-domain antibodies generally do not produce neutralizing antibody responses, but repeated doses of fusion bivalent or multivalent single domain antibodies will result in stronger the immunogenicity is produced, which affect the therapeutic effect. Recently, some researchers have carried out humanization of camellia single-domain antibodies, but whether the immunogenicity of this humanized VHH is smaller than that of camellia single-domain antibodies remains to be confirmed. Despite this, the study of single-domain antibodies as small molecule therapeutic antibodies will continue, and it is believed that in the near future, it will be able to play a huge role in the clinical treatment of various diseases.